Consider modern medicine’s approach to disease. To kill a cancerous tumor, we flood the entire body with toxic chemotherapy, a “shotgun” blast that ravages healthy cells alongside the diseased ones. To fight an infection, we swallow a pill that circulates through our entire system to reach one localized spot. It’s effective, but it’s imprecise. Now, imagine a different approach. Imagine injecting an army of a trillion microscopic robots, each smaller than a blood cell, programmed with a single mission: to hunt down cancer cells and destroy them, to deliver drugs with pinpoint accuracy, or to perform surgery on a single blocked artery. This is the promise of nanotechnology in medicine, and these microscopic medics are rapidly moving from science fiction to scientific fact.
Table Of Content
What Exactly Is a Nanobot? From Sci-Fi to Reality
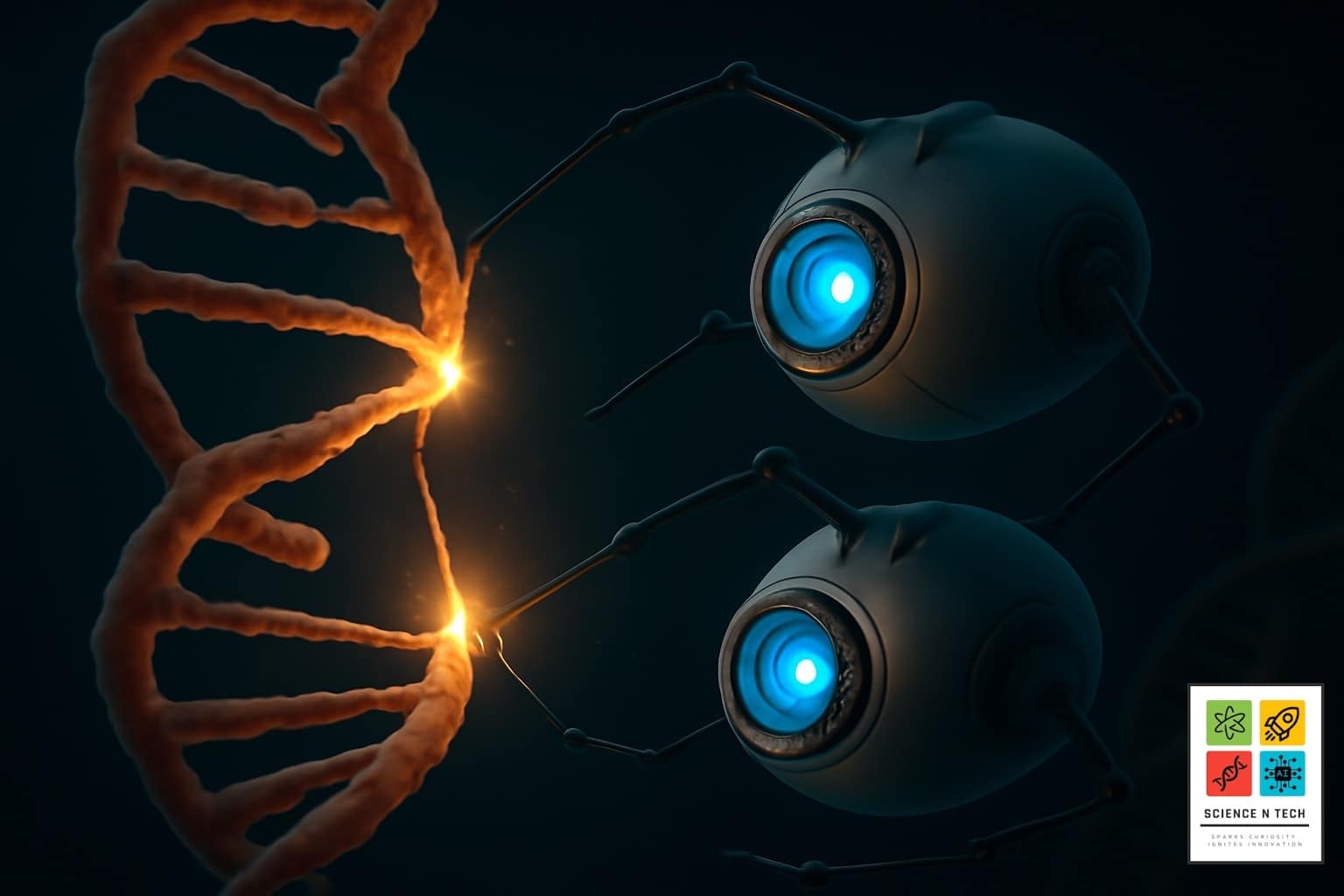
When we hear “nanobot,” we might picture a tiny, metallic robot with gears and propellers, shrunken down to an impossible size. The reality is both more subtle and more elegant. A nanobot is any robotic device operating at the nanoscale (a nanometer is one-billionth of a meter). At this scale, scientists aren’t building with metal and wires; they’re building with the molecules of life itself.
The leading “real-world” nanobots are built from DNA. Through a technique called DNA origami, scientists can fold long strands of DNA into specific, three-dimensional shapes. They can create a hollow box with a lid, a cage, or a barrel. This DNA structure acts as the nanobot’s body, capable of carrying a payload—like a potent dose of a chemotherapy drug.
The “brain” of this nanobot is a set of molecular triggers. The DNA box can be designed with “locks” made of special DNA sequences called aptamers. These locks are programmed to open only when they encounter a specific target protein found exclusively on the surface of a cancer cell. This means the nanobot can circulate harmlessly through the entire body, ignoring healthy tissue. But upon finding its target, it unlocks, opens up, and delivers its deadly cargo directly to the cancer cell, leaving everything else untouched.
The Missions: What Could Nanobots Do Inside Us?
The potential applications of these nanoscopic machines are poised to transform every aspect of healthcare, moving us from an era of treatment to one of pre-emption and precision.
Targeted Drug Delivery: This is the most developed application. By loading nanobots with powerful drugs, we can attack diseases at their source without collateral damage. This would mean drastically reducing the debilitating side effects of treatments like chemotherapy and using drugs that were previously considered too toxic for systemic use.
Early Disease Detection: Imagine nanobots acting as tiny patrol guards in your bloodstream. These “nanosensors” could be designed to search for the faintest chemical traces of disease—the specific proteins shed by a tiny, nascent tumor or the early signs of plaque forming in an artery. Upon detecting these signals, they could send a report to an external device like a smartwatch, alerting you to a disease years before any symptoms appear.
Precision “Nanosurgery”: This is the more futuristic, but awe-inspiring, vision. Researchers are designing nanobots that can perform physical tasks. For example, tiny, propeller-driven bots guided by external magnetic fields could travel upstream through arteries to break up blood clots that cause strokes. Others could identify and destroy individual bacteria or viruses, offering a solution to antibiotic-resistant superbugs.
A surprising fact: The vision of nanomedicine was first proposed by Nobel Prize-winning physicist Richard Feynmanin his legendary 1959 lecture, “There’s Plenty of Room at the Bottom.” He theorized about the possibility of creating nanoscale machines and famously imagined a future where you could “swallow the doctor.”
The Hurdles on the Nanoscale
While the promise is immense, sending a trillion robots into the human body comes with incredible challenges.
- Power and Propulsion: How do you power a machine smaller than a cell? Some nanobots are designed to be passive, simply flowing with the blood. Others are propelled by external forces like magnetic fields or ultrasound. Ingeniously, some are powered by chemistry—tiny rockets coated in zinc that react with stomach acid to produce hydrogen gas bubbles, pushing them forward.
- Biocompatibility: The human immune system is designed to attack any foreign invader. Nanobots must be built from materials that are either ignored by the immune system (like DNA) or are coated in a biological “stealth cloak.”
- Control and Removal: Once their mission is complete, what happens to them? The most elegant solution is to build them from biodegradable materials. DNA nanobots, for instance, simply break down and are recycled by the body’s natural processes within a few days.
Another little-known fact: The first majorly successful trial of nanobots in a living mammal has already happened. In a 2018 study published in Nature Biotechnology, researchers from Arizona State University injected DNA nanobots into mice with cancerous tumors. The nanobots successfully sought out the tumors and delivered a drug that triggered blood clotting, cutting off the tumor’s blood supply and causing it to shrink and decay without harming the host mouse.
The era of nanoscale medicine is no longer a distant dream. While the autonomous nanosurgeon from science fiction is still decades away, the first generation of microscopic medics is already here, promising to make medicine smarter, safer, and more precise than ever before.
As we prepare to unleash these tiny doctors into our bodies, we are creating a new paradigm of healthcare from the inside out. What will medicine look like when our treatments are smaller than our cells, and what does it mean to be “healthy” in a world where disease can be stopped before it even begins?
References
- Feynman, R. P. (1960). There’s Plenty of Room at the Bottom. Engineering and Science, 23(5), 22-36.
- Li, S., Jiang, Q., Liu, S., et al. (2018). A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nature Biotechnology, 36, 258–264.
- Douglas, S. M., Bachelet, I., & Church, G. M. (2012). A logic-gated nanorobot for targeted transport of molecular payloads. Science, 335(6070), 831-834.
- Wang, J. (2009). Can Man-Made Nanomachines Compete with Nature Biomotors? ACS Nano, 3(1), 4-9.
- Service, R. F. (2018, February 12). DNA ‘robots’ successfully treat cancer in mice. Science.